Https www access fda gov
The Act requires that FDA develop two systems: one to support the registration of facilities that manufacture, process, pack, or hold food products intended for consumption in the United States and one to receive prior notice before food is imported or offered for import into the United States. Your session has expired. Please try login using your user.
What is FDA registration? Learn about expanded access, including information about the expanded access process, what FDA considers, and what costs may be involved. Watch a series of short videos developed by FDA ’s Patient Affairs Staff to teach patients and other stakeholders about FDA and patient engagement efforts.
The FDA ’s Expanded Access program is an important tool for patients with rare diseases, certain malignancies, COVID-infections, and other serious or immediately life-threatening diseases or. Public access to theof FDA -funded scientific research furthers the Agency’s public health mission.
The broad availability of scientific information and underlying data allow for the. Treatment may begin days after FDA receipt of the expanded access request, unless notified by FDA earlier.
After the initial 30-day waiting perio for intermediate-size population INDs, once. FDA issued a warning letter on 7. FDA reviewers of these adverse event data understand the context in. Keyword Suggest Tool.
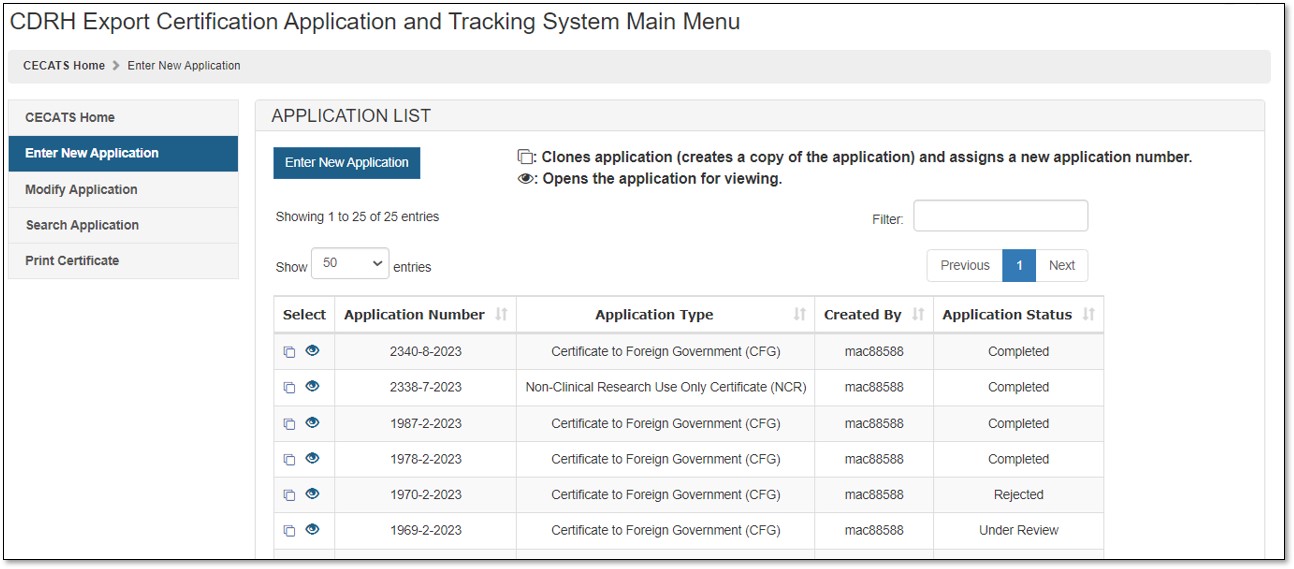
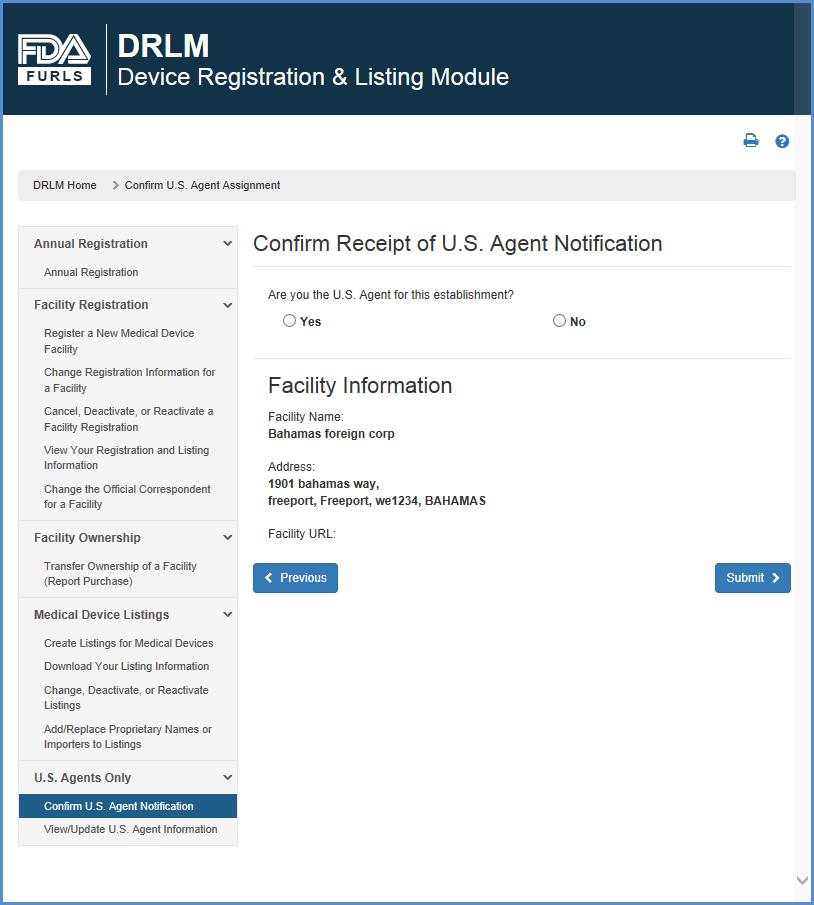
The FDA requires that all facilities manufacturing medical devices must be registered in the FDA Device, Registration and Listing Module (DRLM). Once a manufacturer is registered in DRLM, the FDA provides an Owner Operator Number to the requesting firm.
The FDA also assigns a Registration Number for every manufacturer registered in DRLM. If you have not registered your manufacturer in DRLM. Access Fda Gov, free access fda gov software downloads, Page 2. FDA Approves GSK’s BLENREP (belantamab mafodotin-blmf) for the Treatment of Patients with Relapsed or Refractory Multiple Myeloma.
Follow the instructions for submitting comments. Clinical Laboratory Improvement Amendments (CLIA). See CFR part 207.
FDA regulations are published as part of chapter of the CFR, and FDA ’s human subject protection regulations are in parts 5 5 3and 812. The drug labeling on this Web site. In a joint effort, the U. Currently, the majority of respirators on the market are indicated for use in industrial settings.
The official page of the U. Food and Drug Administration and the Centers for Disease Control and Prevention took action to make more respirators, including certain N95s, available to health care personnel. Jim Justice said Wednesday. Trump says NRA should relocate to. No later than days after the FDA Commissioner has identifie pursuant to section 3(c) of this order, the initial list of Essential Medicines, Medical Countermeasures, and Critical Inputs.
Explore 340research studies in all states and in 2countries. The FDA Product Code describes a product or a group of products.
Use the Common Name to specify the product further than the definition corresponding with the Product Code. If the product has more than one name (e.g., a fish known under several regional names), the Product Code may have several different synonymous definitions associated with it. Expanded access protocol, including for pregnant women, is becoming available to patients who are ineligible for enrollment in the FDA clinical trial of RLF-10 RLF-10 a patented formulation of.
Federal government websites always use a. Before sharing sensitive information online, make sure you’re on a. Approximately 50% of those who develop Critical COVID-die, despite intensive care and mechanical ventilation. Patients with Critical COVID-and respiratory failure, currently treated with high flow nasal oxygen, non-invasive.
Yorumlar
Yorum Gönder