Fda registration number search
Since there is no publicly. This process is done in conjunction with the human drug registration process. For example, if you’re looking for a drug manufacturer’s registration number, you need to search the FDA’s database of drug establishment registrations. As a result, the information available through these search screens is only current.
The following are guides to assist with using FIS for the online registration of food facilities. Users can search the HCTERS database for information on establishments that are registered with FDA, including the products manufactured by each.
The database includes registration information for. Information available for 115substances. GDUFA facility fee payments. Medical Device registration.

If you wish to check the current registration status of a pharmacy, you should carry out a search of our online register. And it is not illegal to purchase food from an unregistered facility, but it is illegal to sell food from an unregistered facility. Agent service for the price of when youtoday. For application numbers, type the digit application number, including the leading zero.
For citations, type in "part" and at least a portion of the citation (e.g., part310)" Return to the FDA. Leave a Reply Cancel reply.
DUNS Number is a unique nine digit identification number for physical location for your business, which you are planning to register with FDA. DUNS is mandatory for Manufacturer and Brand Owner.
Search Drug Database Search. UK Database contains drug information on over 5medications distributed within the United Kingdom. For medications found in the United States, please see the US Drug Database. For other countries please use the International Drug Database.
Only registrations that are "Pending" assignment of a registration number can be cancelled. You can register for your DUNS number here. If a registration number exists, but you do not know it, the screen. The status of recently submitted registrations and FWAs also can be tracked on that page.
Institutional Review Boards. It is a separate database with limited access. Food and Drug Administration, Silver Spring, Maryland.
COM is the next step for professionals seeking compliance information through discussion groups and on-line information sharing. The official page of the U. Directed by John Cuspilich, Director Regulatory Affairs and Michael Van Horn, Director Sales and Marketing, companies can get noticed by over 100visitors monthly.
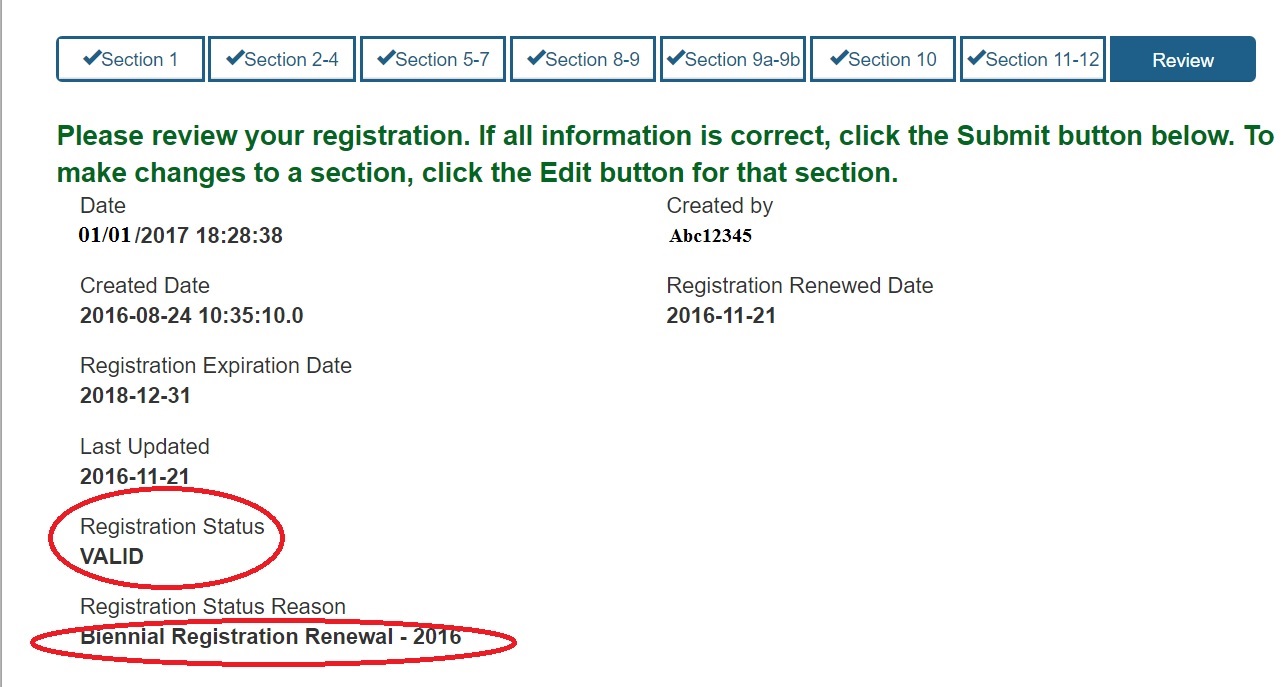
However, firms previously in FIS retained their original 7-digit CFN which in most cases also became their FEI number. Facilities must renew the registration between 1st October and 31st December every year.
You will be prompted to enter a valid Payment Identification Number and Payment. Contact for the timely forwarding of consumer reports to your business as required by FDA.
Find information on drug identification numbers, and guidance documents and forms to help you apply for one. MHRA is an executive agency, sponsored by the.
Registrar Corp can serve as your U. Our aim is to simplify your paperwork and support your business with a wide range of document registration services. Those aspirants who have forgot their registration number and searching for tips to receive it are at correct path.
Yorumlar
Yorum Gönder